Luby Law Firm fights for you when you are suffering from an illness or injury due to an unsafe medical device or product.
Fighting for you and your family when you are suffering from an illness or injury due to an unsafe medical device or product, environmental exposure following potentially defective medical devices, implants, and commercial product. The Luby Law Firm handles and prosecutes mass tort claims, either through individual or collective prosecution. Often, it is our preference to file claims individually in State Court and sometimes in the U.S. District Courts through a process called multi-district litigation.
Mass Tort

Hernia Mesh Lawsuit
If you have suffered from complications from a hernia mesh product, consult with a medical professional and seek legal counsel.
Representing Victims Harmed by Hernia Mesh Complications
There are a variety of hernia mesh products available, though surgeons usually use polypropylene, which is a sterile and woven synthetic plastic-like material. The mesh is applied in the form of a patch, which is placed under or over the weakened area, or inserted as plug. Unfortunately, this treatment has resulted in a plethora of complications, harming many patients.
What is a Hernia?
Hernias commonly occur in the abdomen, though they can also appear in the upper thigh, belly button, and groin. It occurs when an organ pushes through an opening in the muscle or tissue that holds it in place. While this condition is not immediately life threatening, it will not go away on its own and requires treatment.
Causes of Hernia Mesh Complications
The answer to this depends on the type of hernia mesh product used. However, many hernia mesh products contain polypropylene, which is also used in pelvic mesh and bladder slings. Despite a material data safety sheet that explicitly warns against the use of polypropylene in permanent body implantations, manufacturers continue to use it as such. As such, the material has a tendency to erode within a patient’s body, causing serious complications. Mesh erosion can require many additional surgeries, hospitalization, systemic infections, and more. There have even been instances of patients’ teeth rotting out.
Common Hernia Mesh Complications
A wide range of injuries can occur due to hernia mesh complications, some of which can have lifelong impacts such as:
- Infections, including sepsis, requiring the surgical removal of the infected hernia mesh
- Adhesions form to connect the bowel to the hernia mesh, causing bowel obstruction
- Abdominal pain, which may be a sign of adhesions, infection, or nerve damage
- Leg, groin, and testicular pain, which can be debilitating
- Painful sexual intercourse
- Testicle removal might be necessary if the mesh erodes far into the spermatic cord
- Diarrhea is an early sign of mesh attaching to the bowel
- Constipation may indicate a bowel obstruction
- Nausea is a sign of adhesions on the bowel or stomach
- Fever might indicate an infection
- Severe headaches are another common sign of infection
- Autoimmune disorders, which may also result in joint aches and pain
Hernia Mesh Products Under Investigation
There are several hernia mesh products that are currently on the market, many of which are mounting a large sum of complaints. Here is a list of hernia mesh products that are either being investigated or facing lawsuits:
- C-Qur by Getinge Group
- Physiomesh by Ethicon
- Proceed by Ethicon
- Kugel by C.R. Bard
- 3DMax by C.R. Bard
- PerFix Plug by C.R. Bard
- Sepramesh by C.R. Bard
- Ventralex ST by C.R. Bard
- Patrietex Composite by Medtronic
- Surgipro by Medtronic
Reach Out to a Skilled Legal Team
If you have suffered from complications from a hernia mesh product, consult with a medical professional and seek legal counsel. You might have a lawsuit on your hands and receiving compensation can help relieve the burden of medical bills associated with any treatment you may require to recover from any injuries caused by the hernia mesh. The sooner you contact a hernia mesh attorney, the faster you can begin to develop your claim and hold those responsible for the damages you have suffered, and the possible future damages that might arise from having used this dangerous medical device.
At Luby Law Firm, we do not believe you should be responsible for the medical bills incurred as a result of a manufacturer’s product. We will fight to insure that you receive the compensation you deserve.

Essure Injury Lawsuit
Have you or a loved one been injured by Essure? Let us fight on your behalf and pursue the just compensation that you deserve.
Pursue Justice with an Essure Injury Attorney
Essure is a device manufactured by Bayer for the purpose of birth control. It is a non-surgical, non-hormonal birth control product that requires a ten-minute insertion procedure and claims to block the fallopian tubes, effectively keeping sperm from fertilizing the eggs. Unfortunately, this device has been connected to severe injuries.
Have you or a loved one been injured by Essure? Let us fight on your behalf and pursue the just compensation that you deserve. We have won millions for our clients and are ready to take on the toughest opponents.
Complications Associated with Essure
Essure is a flexible device that is inserted into the patient’s fallopian tubes. When this device malfunctions, serious abdominal injuries can result.
Essure has been connected to the following complications:
- Ectopic pregnancies
- Expulsion of the device
- Device migration or “loss” of device
- Allergic reactions
- Chronic pelvic pain
- Autoimmune responses
- Tearing or puncturing of pelvic organs
- Need for follow-up surgeries or hysterectomy
- Obesity
To date, thousands of Essure patients have reported similar complications and numerous lawsuits have been filed that are related to the Essure birth control device.
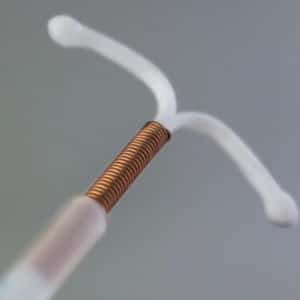
Paraguard Lawsuit
If you or a loved one have suffered an injury from a Paragard IUD device, please contact our law firm today as you may be entitled to financial compensation.
Our law firm is currently evaluating claims for injuries resulting from the Paragard IUD device. Recent FDA studies have reported the ParaGard IUD could break or fracture. This can result in perforation of the uterus or other injuries requiring invasive surgery to remove. Reports indicate the device can also lead to complications such as infections, scarring, or loss of reproductive health. If you or a loved one have suffered an injury from a Paragard IUD device, please contact our law firm today as you may be entitled to financial compensation.
What is a ParaGard IUD?
Paragard IUD is a hormone free device implanted in the uterus to provide long term protection against pregnancy. The Paragard IUD is unique from other IUD devices in preventing fertilization, as it was manufactured with copper that produces an inflammatory reaction that acts like a spermicide to prevent pregnancy. The Paragard IUD was approved by the FDA in 1984 and easily implanted in patients with a simple doctor’s visit. The Paragard IUD was marketed as a reversible birth control device.
Paragard IUD Warnings
Recent studies have reported injuries and complications from the Paragard IUD device. The manufacturer of the Paragard IUD has recently stated the device may become difficult to remove “because it is stuck in the uterus”. They added that “Surgery may sometimes be needed to remove Paragard”.
Patients are reporting that upon removal the Paragard IUD device had broken inside the uterus or missing the copper coil typically wound around the plastic T-shaped base. If the device is found to have broken in the uterus previously or during removal, the patient could then be required to have invasive surgery to remove the broken pieces. A full hysterectomy may be required in some cases. Broken pieces of the Paragard IUD can lead to perforation of the uterus wall. These perforations can cause additional complications such as scarring, infections, or damage to other nearby organs. Invasive surgery will be required to correct injuries from perforation of the uterus.
Paragard Injuries
- Perforation of the uterus
- IUD becomes embedded in the uterus
- Device breakage (copper wire) requiring removal surgery
- Infections or scarring
- Loss of reproductive health
Consult a Doctor on Medical Issues
Our law firm does not intend, by this web site or otherwise, to dissuade anyone from taking the advice of medical professionals and their doctors. Please consult your doctor, not your lawyer, on matters relating to your health.
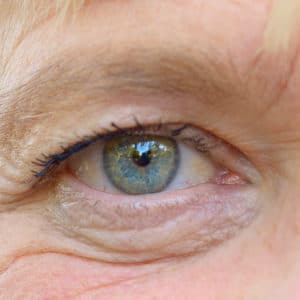
Elmiron Lawsuits: ID Drug Linked to Eye Damage
If you or a loved one took Elmiron and later suffered from vision issues, you may be entitled to compensation.
Elmiron lawsuits allege that the drug maker did not disclose the link between Elmiron use and serious vision damage and failed to warn patients about this risk.
In June 2020, the drug maker updated the product labeling to inform users of potential vision problems, though this warning comes too late for those already suffering from eye damage.
If you or a loved one took Elmiron and later suffered from vision issues, you may be entitled to compensation.
Elmiron’s Potentially Permanent Side Effects
Elmiron, a prescription drug used to treat bladder pain and discomfort associated with interstitial cystitis (IC), has come under fire for its link to serious eye damage. Elmiron users across the United States claim that the drug may cause many vision issues.
Elmiron may be linked to the following diseases:
- Macular degeneration
- Pigmentary maculopathy
- Retinal maculopathy
Other Elmiron side effects may include:
- Blurred vision
- Difficulty reading
- Distorted vision
- Eye pain
- Issues adjusting to darkness
- Vision disturbances
- Vision loss
What Is Elmiron Used For?
Approved by the U.S. Food and Drug Administration (FDA) in 1996, pentosan polysulfate sodium (Elmiron) is the only FDA-approved oral medication designed to treat bladder pain and discomfort associated with interstitial cystitis (IC).
IC, also known as “painful bladder syndrome,” is a chronic bladder condition with no known cure.
If you or a loved one used this IC drug and have since developed one of the vision impairments listed above, you may be eligible to file an Elmiron lawsuit.

3M’s Combat Earplugs Lawsuit
If you used 3M Dual-Ended Combat Arms Earplugs while in the military and now suffer from tinnitus or hearing loss, contact us for a free, no-risk consultation.
Hundreds of veterans are suing 3M Company after developing tinnitus and hearing loss while using defective 3M combat earplugs. As with most large product liability lawsuits, there are a lot of questions and confusion surrounding the earplugs themselves, their defect, who is affected, and how to file a claim for financial compensation.
With this in mind, The Luby Law Firm has developed the following guide answering some of the most frequently asked questions concerning 3M’s combat earplugs. If you have any additional questions, do not hesitate to contact us directly for a free consultation.
What Earplugs Are Covered by the 3M Lawsuits?
Currently, lawsuits are dealing strictly with 3M’s Dual-Ended Combat Arms Earplugs (CAEv2). The earplugs were originally developed by Aearo Technologies and later manufactured by 3M Company following its acquisition of Aearo Technologies in April 2008.
The 3M earplugs are known as “selective attenuation earplugs” and consist of two inverted cones, one black and one yellow, connected by a short stem. This dual-ended design is meant to provide wearers with two different levels of protection: one end (or cone) works like a traditional earplug while the other end, referred to as the “open” end, allows the wearer to hear quieter sounds while still attenuating loud noises.
The general idea was that troops could use the open end to hear speech, commands, and orders while still being protected from louder sounds like gunfire and explosions.
What is the Problem with the 3M Earplugs?
According to court documents, the stem that connects the two ends of the 3M Dual-Ended Combat Arms Earplugs is too short and prevents the earplugs from sitting correctly in the ear. As such, loud noises are able to slip through, past the earplugs, and cause hearing damage. Additionally, the 3M earplugs can “loosen in the wearers ear, imperceptibly to the wearer and even trained audiologists visually observing the wearer, thereby permitting damaging sounds to enter the ear canal by traveling around the outside of the earplug.”
To make matters worse, the U.S. Justice Department alleges that both 3M and Aearo Technologies, the original manufacturer of the earplugs, knew about the defect as early as 2000 and actually manipulated test results to make it appear that the devices met government standards. They then proceeded to sell the faulty earplugs to the U.S. government, placing personal profit over the safety and health of U.S. troops.
How Many Troops Were Affected by the Defective Earplugs?
While it is still unclear how many troops suffered hearing damage due to the defective earplugs, potentially millions of service members were issued the earplugs.
Aearo Technologies was able to secure a contract with the U.S. government in 2003 becoming the sole provider of combat earplugs for the U.S. military. The contract moved to 3M Company as part of its acquisition of Aearo technologies in 2008. Consequentially, the defective earplugs were issued to troops deployed in combat zones around the world from 2003 to 2015.
How Do I Know If I Used Defective Military Earplugs?
The defective earplugs were issued to U.S. military members deployed in the following combat zones from 2003 to 2015:
- Afghanistan
- Iraq
- Pakistan
- Somalia
- Libya
- The Indian Ocean
Further, it appears soldiers also used the earplugs during training. This means service members may have been exposed to damaging sounds, even if they were not deployed in combat zones.
How Was the Defect Discovered?
News of the problem broke in July 2018 when 3M agreed to pay the U.S. Department of Justice $9.1 million to settle allegations that it knowing sold the defective earplugs to the U.S. military. The settlement was part of a False Claims Act lawsuit against the company. 3M did not admit any wrongdoing as part of the settlement.
Unfortunately, none of the $9.1 million paid by 3M will go to service members and veterans who developed tinnitus, hearing loss, or hearing damage, leaving the individuals affected the most to seek methods of recovery and compensation on their own.
Have the Defective Earplugs Been Recalled?
No. 3M discontinued production of the earplugs in 2015, but the earplugs were not ever actually recalled. As a result, it is likely the defective pairs are still being used by soldiers and sold by other vendors.
What Are Symptoms of Military-Related Hearing Damage?
Hearing loss is the most common disability among veterans. Frequent and persistent exposure to damaging levels of sound such as gunfire, aircraft noise, loud machinery, and explosions mean service members experience hearing loss and tinnitus at a much greater frequency than the average adult. In fact, in 2003, the Department of Veterans Affairs (VA) reported that auditory system injuries, including full or partial hearing loss and tinnitus, were the third most common type of service-connected disability.
As of 2017, the top two service-connected disabilities for all compensation recipients were tinnitus and hearing loss.
According to the Department of Defense, the most common symptoms of combat-related hearing problems include:
- Buzzing or ringing in the ears
- Difficulty hearing someone talking three feet away
- Difficulty understanding what people are saying
- A feeling of “fullness” in the ears after leaving a noisy area, such as a concert venue
However, hearing damage is complicated and symptoms can vary depending on the noise levels the sufferer was exposed too. Tinnitus, for example, is not limited to sounds of ringing and buzzing. In fact, some sufferers describe their tinnitus as hissing, clicking, roaring, or whistling.
Other symptoms of hearing loss include:
- Muffling of speech and other sounds
- Difficulty understanding words, especially against background noise
- Trouble hearing consonants
- Frequently asking others to speak more slowly, clearly, or loudly
- Withdrawal from conversations
- Needing to turn up the volume of the television or radio
- Difficulty sleeping
- Difficulty concentrating
What Kinds of Damages Can I Seek?
Damages are not restricted to your physical injury, and damages may extend to include other facets of every day life that are affected, lost, or diminished. Among the most common damages sought in 3M earplug claims are:
- Injury
- Pain and suffering
- Loss of consortium
- Medical expenses
- Lost wages
- Loss of earning potential
What Should I Do If I Suffered Hearing Loss While Using 3M Military Earplugs?
If you used 3M Dual-Ended Combat Arms Earplugs while in the military and now suffer from tinnitus or hearing loss, contact Luby Law Firm for a free, no-risk consultation. Our attorneys can help answer your questions concerning your use of 3M military earplugs, the hearing loss and damage you may have suffered, and your options for filing a 3M earplugs claim and seeking financial consolation.
For the past 25 years, our experienced product liability attorneys have handled a multitude of defective product cases and have litigated against some of the largest companies in the world, including General Motors as part of their defective ignition switch fiasco. Our extensive legal and financial resources allow us to provide our clients with truly dynamic legal representation.
Let us help you recover the compensation you are entitled to. Remember, Luby Law Firm works on a contingency fee basis – this means you do not pay us a dime unless and until we have won your case.

Zantac Lawsuit
If you or a loved one took Zantac and developed cancer, you may be entitled to substantial compensation.
What is Zantac?
Zantac is a popular heartburn medicine available over the counter. People often take Zantac to relieve the symptoms of acid indigestion or sour stomach. When the drug works properly, it inhibits the amount of acid created by the stomach to prevent and relieve heartburn. Zantac was first sold in the United States in 1983 and became the first drug to total $1 billion in sales. It remains a widely popular heartburn drug.
Recent tests have shown that the drug may contain dangerous levels of NDMA, more than 26,000 times the permissible intake level.
What is NDMA?
NDMA is a probable human carcinogen. NDMA is a volatile, yellow liquid that emits toxic fumes. It is a byproduct of several industrial processes and present in some foods. Its primary use is in cancer research, where scientists use the chemical to cause cancer in lab animals.
Besides Zantac and other ranitidine drugs, NDMA has also been found in some versions of valsartan, a blood-pressure drug.
You May Have a Zantac Claim if
The U.S. Food and Drug Administration announced it is requesting manufacturers withdraw all prescription and over-the-counter (OTC) ranitidine drugs from the market immediately. This is the latest step in an ongoing investigation of a contaminant known as N-Nitrosodimethylamine (NDMA) in ranitidine medications (commonly known by the brand name Zantac). The agency has determined that the impurity in some ranitidine products increases over time and when stored at higher than room temperatures and may result in consumer exposure to unacceptable levels of this impurity. As a result of this immediate market withdrawal request, ranitidine products will not be available for new or existing prescriptions or OTC use in the U.S.
“The FDA is committed to ensuring that the medicines Americans take are safe and effective. We make every effort to investigate potential health risks and provide our recommendations to the public based on the best available science. We didn’t observe unacceptable levels of NDMA in many of the samples that we tested. However, since we don’t know how or for how long the product might have been stored, we decided that it should not be available to consumers and patients unless its quality can be assured,” said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research. “The FDA will continue our efforts to ensure impurities in other drugs do not exceed acceptable limits so that patients can continue taking medicines without concern.”
NDMA is a probable human carcinogen (a substance that could cause cancer). In the summer of 2019, the FDA became aware of independent laboratory testing that found NDMA in ranitidine. Low levels of NDMA are commonly ingested in the diet, for example NDMA is present in foods and in water. These low levels would not be expected to lead to an increase in the risk of cancer. However, sustained higher levels of exposure may increase the risk of cancer in humans. The FDA conducted thorough laboratory tests and found NDMA in ranitidine at low levels. At the time, the agency did not have enough scientific evidence to recommend whether individuals should continue or stop taking ranitidine medicines, and continued its investigation and warned the public in September 2019 of the potential risks and to consider alternative OTC and prescription treatments.
New FDA testing and evaluation prompted by information from third-party laboratories confirmed that NDMA levels increase in ranitidine even under normal storage conditions, and NDMA has been found to increase significantly in samples stored at higher temperatures, including temperatures the product may be exposed to during distribution and handling by consumers. The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA. These conditions may raise the level of NDMA in the ranitidine product above the acceptable daily intake limit.
With today’s announcement, the FDA is sending letters to all manufacturers of ranitidine requesting they withdraw their products from the market. The FDA is also advising consumers taking OTC ranitidine to stop taking any tablets or liquid they currently have, dispose of them properly and not buy more; for those who wish to continue treating their condition, they should consider using other approved OTC products. Patients taking prescription ranitidine should speak with their health care professional about other treatment options before stopping the medicine, as there are multiple drugs approved for the same or similar uses as ranitidine that do not carry the same risks from NDMA. To date, the FDA’s testing has not found NDMA in famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid) or omeprazole (Prilosec).
In light of the current COVID-19 pandemic, the FDA recommends patients and consumers not take their medicines to a drug take-back location but follow the specific disposal instructions in the medication guide or package insert or follow the agency’s recommended steps, which include ways to safely dispose of these medications at home.
The FDA continues its ongoing review, surveillance, compliance and pharmaceutical quality efforts across every product area, and will continue to work with drug manufacturers to ensure safe, effective and high-quality drugs for the American public.
The FDA encourages health care professionals and patients to report adverse reactions or quality problems with any human drugs
If you or a loved one took Zantac and developed cancer, you may be entitled to substantial compensation. Contact Luby Law Firm for a free and confidential case evaluation and find out how you can join the Zantac lawsuit. Complete our contact form or call us at (314) 421-5829.
The U.S. Food and Drug Administration (FDA) has warned that ranitidine medicines, including heartburn drugs sold under the name “Zantac,” have an impurity that may cause cancer. In mid-September, the FDA published a statement to alert patients and doctors that the agency had found dangerous levels of N-nitrodimethylamine (NDMA) in Zantac and related products.
People who have taken Zantac are now suing the manufacturers of the drug, Sanofi and Boehringer Ingelheim. Plaintiffs say that the drug manufacturers knew or should have known that Zantac contained high levels of NDMA, a chemical linked to cancer. If you or a loved one took Zantac and then developed cancer, filing a lawsuit may help you pay for medical bills, lost wages, and other costs associated with your suffering. Lawsuits can also help send a strong message to big pharmaceutical companies that they should ensure that their products do not cause harm before selling them to the public.
What are the symptoms of NDMA exposure?
NDMA exposure can cause liver damage, and studies have linked the chemical to a variety of cancers. Overexposure to NDMA has been linked to the following symptoms:
- Headache
- Fever
- Nausea
- Jaundice
- Vomiting
- Cramps
- Dizziness
If you have experienced any of the above symptoms after taking Zantac or a Zantac-like ranitidine drug, you should consult a medical professional. Do not quit taking any medication you have been prescribed without first consulting your doctor. Scientific studies have also linked NDMA exposure to a variety of cancers, including the following:
- Bladder cancer
- Stomach cancer
- Small intestine cancer
- Colorectal cancer
- Esophageal cancer
- Liver cancer
- Prostate cancer
- Pancreatic cancer
- Leukemia
- Non-Hodgkin’s Lymphoma
- Multiple myeloma
What does the Zantac lawsuit say?
Plaintiffs accuse Sanofi and Boehringer Ingelheim of creating and promoting a product that they knew or should have known could cause cancer. It is unlawful to conceal safety defects in a product and market it to the public as safe. Plaintiffs accuse the manufacturers of Zantac of deceiving the public about the safety of Zantac and hiding the fact that the drug may contain a probable human carcinogen.
Has there been a recall of ranitidine?
Yes. The FDA has announced the manufacturers of generic Zantac-like drugs have chosen to voluntarily recall their medicines available over the counter. You may have seen news stories that say that major retailers like CVS, Walgreens, Rite Aid and Walmart have pulled ranitidine drugs from their shelves at the suggestion of manufacturers of Zantac-like generic drugs. Some retailers have chosen to stop selling ranitidine products altogether after the FDA’s announcement that the drugs could contain a probable human carcinogen.
Do I have a Zantac case?
Contacting a lawyer is the best way to determine whether you have a Zantac case. A lawyer is in the best position to evaluate your facts and circumstances and help you secure your maximum recovery. The experienced lawyers and staff at the Luby Law Firm can help. We will listen to your story and assist you in deciding your legal path forward. Our case evaluations are free and confidential. Contact us today for more information on the Zantac lawsuit by completing our contact form or calling us at (314) 421-5829.
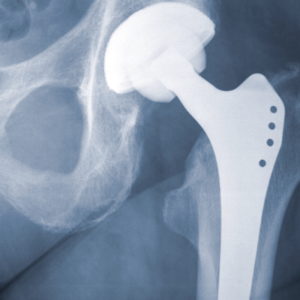
Defective Hip Implantment Lawsuit
Take legal action against negligent manufacturers and product representatives.
The last thing you want to find out after hip replacement surgery is that the replacement part you received may prematurely fail and cause severe pain and other serious injuries. If you or a loved one is experiencing infection, swelling, implant dislocation or other painful side effects from a metal hip replacement, our lawyers are prepared to help you seek justice.
Luby Law Firm represents patients and are reviewing new claims in metal-on-metal hip replacement cases, including those involving:
- DePuy® Orthopaedics ASR™ XL Acetabular Hip Replacement System (implanted between 2005 and 2010)
- Stryker® Rejuvenate
- Stryker® ABG II Modular-Neck Hip Stems
- Stryker® LFIT V40 Femoral Head
- Wright Medical Technology Hip Replacements (Conserve®, Dynasty®, Profemur® and Lineage® line of products)
- Also, Luby Law Firm is reviewing new defective hip cases involving other manufacturers
Metal-on-metal hip devices were originally thought to be a more durable alternative to ceramic or plastic models, but some studies have shown that they may deteriorate faster, expose patients to high levels of metals such as cobalt and chromium, and fail at a higher rate than older implants.
In fact, approximately one in 12 recipients of metal-on-metal devices may require corrective surgery within five years of implantation.
The last thing you want to find out after hip replacement surgery is that the replacement part you received may prematurely fail and cause severe pain and other serious injuries. If you or a loved one is experiencing infection, swelling, implant dislocation or other painful side effects from a metal hip replacement, our lawyers are prepared to help you seek justice.
Metal-on-metal hip devices were originally thought to be a more durable alternative to ceramic or plastic models, but some studies have shown that they may deteriorate faster, expose patients to high levels of metals such as cobalt and chromium, and fail at a higher rate than older implants.
Side effects may include:
- Blood test results that show high levels of chromium and cobalt caused by metal debris
- Bone loss caused by device loosening
- Clicking, popping or grinding in the area of the hip implant
- Difficulty and pain when walking, standing or carrying weighted objects
- Dislocation or loosening of the implant
- Earlier than normal failure of the hip replacement
- Fractured hipbone
- Infection
- Leg length discrepancy
- Metallosis (metal flakes released into the bloodstream causing metal toxicity, pseudotumors, rash, necrosis or cardiac complications)
- Rash, indicating necrosis (cell death around the implant manifesting as a rash and eventually causing the loss of local soft bone and tissue)
- Osteolysis (wearing down and thinning of bones causing pain in the surrounding tissue and potential bone breakage)
- Pseudotumors (a mass of inflamed cells that resembles a tumor but is actually collected fluids)
- Severe pain
- Swelling of hip, thigh or groin area
- Tissue inflammation
Any of the above symptoms or related conditions that result in revision surgery to remove a hip device.
If you received a metal hip implant and have experienced any of the symptoms listed above, contact your physician immediately. Also, do not release any medical information to the hip replacement manufacturer representatives or sign a release before speaking to a lawyer. If you are told that you need hip revision surgery, make sure that the removed component parts are preserved and consult your counsel to ensure they are saved correctly.
If you or a loved one have experienced adverse side effects from a metal-on-metal hip implant, please contact Luby Law Firm at (314) 421-5829 for more information or to discuss a potential claim.

Talcum Powder Lawsuit
Repeated use of talcum powder in the genital area may increase a woman’s risk of ovarian cancer. Women who have developed ovarian cancer after using talc-based baby powder are eligible for a free case review and may be entitled to compensation.
Repeated use of talcum powder in the genital area may increase a woman’s risk of ovarian cancer. Women who have developed ovarian cancer after using talc-based baby powder are eligible for a free case review and may be entitled to compensation.
Talc Powder Breaking News
On May 19, 2020, Johnson & Johnson announced that it will discontinue sales of talc-based Johnson’s Baby Powder in the United States and Canada.
The medical and cosmetic goods giant made its decision following declining sales and the continued rise in lawsuits claiming its baby powder caused cancer. The baby powder lawsuits alerted many Americans to the link between the use of Johnson & Johnson talc-based products and ovarian cancer.
This announcement came after U.S. Food and Drug Administration (FDA) testing found asbestos contamination in select Johnson & Johnson products in 2019.
With dozens of lawsuits mounting against Johnson & Johnson, many are speculating whether the decision to pull one of their best-known products is really about diminishing sales.
It has become all too possible that the corporation is seeing the writing on the wall — and that the danger of their products have been exposed to the public and there’s no turning back.
Could this lead to them accepting liability for their actions? Will they continue to hide behind a shield of virtue, or will they do the right thing?
For the consumers’ sake, hopefully Johnson & Johnson is realizing the end game is near.
Cancer Victims Are Filing Talcum Powder Lawsuits
Johnson & Johnson® has known about the dangers associated with the continuous use of talcum products since the 1970s. However, they will not add a warning label to the product packaging or ads.
The company generated a memo on March 3rd, 1975 instructing its managers to stop all internal studies into the safety of talc products in order to avoid uncovering any information that might be damaging or embarrassing. Company policy was to only initiate studies as a reaction to confrontation.
Johnson & Johnson Ovarian Cancer Lawsuits
Johnson & Johnson lost their first talcum powder case in February 2016 when an Alabama woman, who developed ovarian cancer from using their baby powder, won $72 Million in compensatory damages.
Since then, there have been several multi-million dollar jury verdicts against Johnson & Johnson talcum powder — in New Jersey, California, Missouri, and across the U.S.
Multi-million dollar talcum powder cancer verdicts include:
- $72 Million awarded by Johnson & Johnson in 2016
- $55 Million awarded by Johnson & Johnson in 2016
- $110 Million awarded by Johnson & Johnson in 2017
- $417 Million awarded by Johnson & Johnson in 2017
- $117 Million awarded by Johnson & Johnson and Imerys Talc America in 2018
In spite of the major jury awards, this is only the tip of the iceberg. Johnson & Johnson alone is currently facing more than 16,000 talc-related lawsuits as consumers who were put at risk by greed and irresponsible business practices ask for justice and compensation in court.
If you or someone you love uses talcum powder, or used it in the past, and have since been diagnosed with ovarian cancer, contact talcum powder cancer law firm, Luby Law Firm, for a free case review. You may be eligible for compensation.
Does Talcum Powder Cause Cancer?
Regular use of talc-based baby powder in the genital area may increase a woman’s chance of developing ovarian cancer by as much as 30%, according to a research report in the Journal of Epidemiology, Biomarkers & Prevention.
Talc is one of the main ingredients in products such as Johnson’s Baby Powder® and Shower to Shower® body powder for women. A naturally-occurring mineral, talc shares chemical similarities with the carcinogen asbestos.
When talcum powder is directly applied to the genital area, talc particles can travel up the vagina, into the fallopian tubes, and then into the ovaries, where the talc molecules can lodge for decades.
Talc can cause inflammation in otherwise healthy tissue and, when chronic, this inflammation may contribute to the development of cancer.
Talcum Powder Ovarian Cancer
While a long history of medical studies has firmly linked repeated use of talc products to an increased risk of ovarian cancer, some ovarian cancers are more likely to develop than others.
The most common talc-related ovarian cancer diagnosis is epithelial ovarian cancer (EOC). Epithelial tumors grow on the outer surface of the ovaries and come in several varieties, all of which are affected by prolonged use of talcum powder, according to an analysis published in the journal Cancer Prevention Research.
Like most ovarian cancers, EOC is difficult to detect during routine gynecological care. It may not show up on a pap smear, and ovarian tumors are difficult to feel during a routine pelvic exam.
Common methods of early EOC detection include:
- Transvaginal ultrasound:Uses sound waves to search for tumors in the uterus, fallopian tubes, and ovaries
- A blood test:Checks for the CA-125 protein, high levels of which are often found in women with ovarian cancer
However, doctors may be unlikely to use either of these methods unless a patient is already showing persistent symptoms of ovarian cancer.
The symptoms of ovarian cancer tend to hide in plain sight, resembling symptoms of other, less dangerous conditions. By the time doctors think to check for ovarian cancer, it has often already started spreading to nearby organs and through the rest of the body.
Ovarian cancer symptoms may include:
- Back pain
- Bloating
- Constant fullness/trouble eating
- Constipation
- Fatigue
- Frequent urination
- Menstrual changes
- Pelvic or abdominal pain
- Stomach swelling
- Weight loss
Any woman experiencing these symptoms daily for more than a few weeks should speak to her doctor as soon as possible for proper screening, according to the American Cancer Society (ACS).
Risks of Talcum Powder for Women Known for Decades
Studies researching the link between talcum powder and ovarian cancer have been published for decades.
In 1982, Harvard Medical School professor Dr. David Cramer published the first scientific study associating talcum powder use on women’s genital area with increased ovarian cancer risk. Subsequent research published through the 1980s and 1990s supported Dr. Cramer’s findings.
A study published in 1992 urged manufacturers to put warning labels on talcum powder products because of the threat to women’s health.
In fact, Johnson & Johnson lawyers admitted in federal court that the company had been aware of the research associating ovarian cancer with talc since the early 1980s, and the corporation intentionally decided not to put warnings on their packaging or in their ads.
Due to the overwhelming scientific evidence and Johnson & Johnson’s refusal to add warning labels, we are currently reviewing cases involving ovarian cancer after using talc-based baby powder.
Get Legal Help for Talcum Powder Ovarian Cancer
If you or a loved one has been diagnosed with ovarian cancer after regular talc use, you may be entitled to compensation.
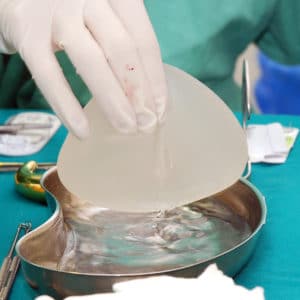
Allegran Lawsuit
If you or a loved one were diagnosed with Large Cell Lymphoma after having Breast Implants, you may be eligible to compensation.
Allergan Breast Implant Recall History
On July 24, 2019, Allergan announced a recall of certain BIOCELL textured breast implants after reports showed a possible link between the implants and a type of non-Hodgkin’s lymphoma.
In December 2018, sales of textured Allergan breast implants were suspended in all 33 countries in Europe as well as Israel, Brazil, and Australia. Canada suspended Allergan’s license for its BIOCELL breast implants in May 2019 after an internal safety review concluded that these implants were linked to an increased risk of cancer. Before the recall, there was enough evidence to halt sales of Allergan’s BIOCELL breast implants in many countries across the world.
Allergan BIOCELL Breast Implants Linked to Cancer
Reports have shown that people with Allergan’s BIOCELL textured products were six times more likely to develop breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) than people with textured implants from other manufacturers.
The World Health Organization officially designated BIA-ALCL as a form of T-cell lymphoma that can develop following breast implantation.
NOTE: The Luby Law Firm is a law firm and cannot provide medical advice. If you have medically related questions or concerns regarding any side effects that you may be experiencing or a recall notice you may have received, please contact your surgeon for more information.
How The Luby Law Firm Can Help
Allergan was recently directed by the FDA to recall certain BIOCELL breast implants due to concerns that they cause cancer, but the recall only covers the cost of replacement implants but does not cover the cost of other damages such as pain and suffering, additional medical expenses, or lost wages.
If you or a loved one were diagnosed with Large Cell Lymphomaafter having Breast Implants, you may be eligible to compensation. Chat with a live representative, submit an inquiry through the form below, or call us (314) 421-5829 today for a free consultation.

RoundUp Lawsuit
Thousands have been ravaged by this dangerous product and The Luby Law Firm looks forward to assisting you with your injury.
Presently in St Louis County, thousands of cases are pending with respect to individuals diagnosed with Non Hodgkins Lymphoma. This devastating disease is the result of prolonged exposure and use to the product Roundup which contains a deadly chemical called glysphophate. The Luby Law Firm is currently representing individuals with the disease in St. Louis County and beyond.
Roundup Weed Killer Lawsuits
In 1970, agricultural giant Monsanto developed glyphosate as a potent herbicide. Monsanto marketed the chemical as Roundup Weed Killer. By 2007, it had become the most used herbicide in the United States. An estimated 1.4 billion pounds of Roundup are used in more than 160 countries each year.
Despite its widespread use, the popular weedkiller has been called into question as a possible health hazard. Thousands of consumers have filed Roundup cancer claims alleging they developed non-Hodgkin’s lymphoma, b-cell lymphoma, leukemia or other forms of cancer after using the weed killer.
The first non-Hodgkin’s lymphoma lawsuit came before a jury in 2018, resulting in a landmark $289 million verdict against Monsanto. A judge reduced the award on appeal, but the Plaintiff, Dewayne Johnson, and his family received $78.5 million.
In 2018, just as these lawsuits were beginning to come before juries, Bayer finalized their acquisition of Monsanto. Moreover recently in California Monsanto was held liable for millions of dollars, however, multiple claims still remain throughout the United States. On August 10, 2018, a San Francisco jury returned with a verdict that awarded Mr. Johnson $289 million ($39 million in compensatory damages and $250 million in punitive damages) in his Roundup case.
Roundup Wrongful Death Lawsuits
In some cases, individuals impacted by repeated Roundup exposure may have already passed away before they or their family members understood the link between Roundup and cancer. Family members of those who passed away from Roundup-associated cancer may be able to file a wrongful death lawsuit.
Roundup Cancer Settlements, Verdicts and Compensation Amounts
In addition to the verdicts some Roundup claims have been settled and others are in the process of being settled, however other remain in litigation. Also a number of new class actions and property damage claims have been filed regarding loss of property and consumer fraud. This process is ongoing and The Luby Law Firm is taking new claims regarding the damage Roundup has caused numerous individuals.
The Evidence Against Roundup & Monsanto
In one recent Monsanto lawsuit, unsealed documents and emails emerged suggesting Monsanto ghost wrote several scientific studies. The emails indicate Monsanto wanted to ensure regulatory bodies, like the EPA, found glyphosate to be safe.
Roundup cancer lawyers claim company executives worked with a former EPA employee to refute claims that glyphosate was dangerous and tried to squash an investigation of the herbicide. Monsanto allegedly received notice of the herbicide’s evaluation months before it was made public, allowing them to prepare for a public relations attack against studies supporting a cancer link.
The Monsanto Documents
Documents uncovered during the discovery process in early Roundup lawsuits have revealed a number of questionable Monsanto actions. The consumer advocate organization US Right to Know obtained most of these documents through public records request and made them available on their website as a public service.
The collection of documents, now known as The Monsanto Documents, show Monsanto used three tactics in attempts to manipulate public perception of Roundup:
- Attempted (and partially succeeded) to manipulate regulatory body classifications of glyphosate. Monsanto actually manipulated the EPA towards its positions with respect to the data
- Manipulated scientific publications about glyphosate and mispresented the information to the public
- Funded “academic” groups who attacked the organic food industry (for attacking glyphosate and genetically modified foods like Roundup Ready crops). Monsanto also hired its owned experts to manipulate the government.
Monsanto and The IARC
The World Health Organization’s (WHO’s) International Agency for Research on Cancer (IARC) reviewed published data investigating the association between glyphosate and non-Hodgkin’s Lymphoma. As a result of its review, IARC classified glyphosate as a probable human carcinogen in March 2015.
Monsanto, possibly anticipating the role IARC’s classification could play in legal proceedings, mounted an anti-IARC campaign. The pro-glyphosate task force developed and executed a number of tactics aimed at discrediting the scientists and regulators involved in IARC’s decision. According to a taped deposition from Sam Murphey, a former Monsanto executive, Monsanto dedicated $16 to 17 million to anti-IARC and glyphosate-related “media relations” in one year alone.
Conclusion
Luby Law Firm currently has handled claims involving Roundup and would be honored to help you and your family with respect to this horrible product. Thousands have been ravaged by this dangerous product and the Luby Law Firm looks forward to assisting you with your injury. If you feel you have a claim please contact us at (314) 421-5829.